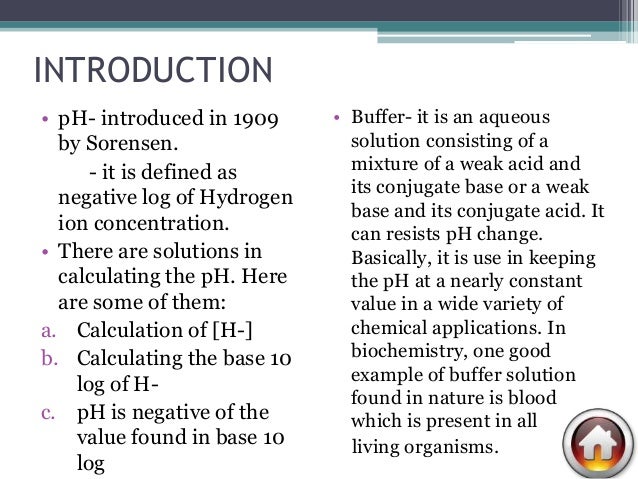
Definition Of Buffer Solution With Example
The K a for acetic acid is 17 x 10 -5. A solution which resists the change in its pH value even on the addition of a small amount of strong acid or base is called a buffer solution or buffer.
An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.

Definition of buffer solution with example. An example of a common buffer is a solution of acetic acid CH 3 COOH and sodium acetate. In other words a buffer is a solution that is able to maintain the pH condition of a solution. One example of a buffer solution found in nature is blood.
A buffer is an aqueous solution that consists of a mixture of a weak acid and its salt acid buffer or a weak base with its salt basic buffer. A buffer solution was made by dissolving 100 grams of sodium acetate in 2000 mL of 100 M acetic acid. A common example would be a mixture of ethanoic acid and sodium ethanoate in solution.
They resist a change in pH upon dilution or upon the addition of small amounts of acidalkali to them. This reforms the weak acid and reduces the amount of H ions in solution. In chemistry buffer solution and examplesIt is a solution containing either a weak acid and its salt or a weak base and its salt which resists changes in pH.
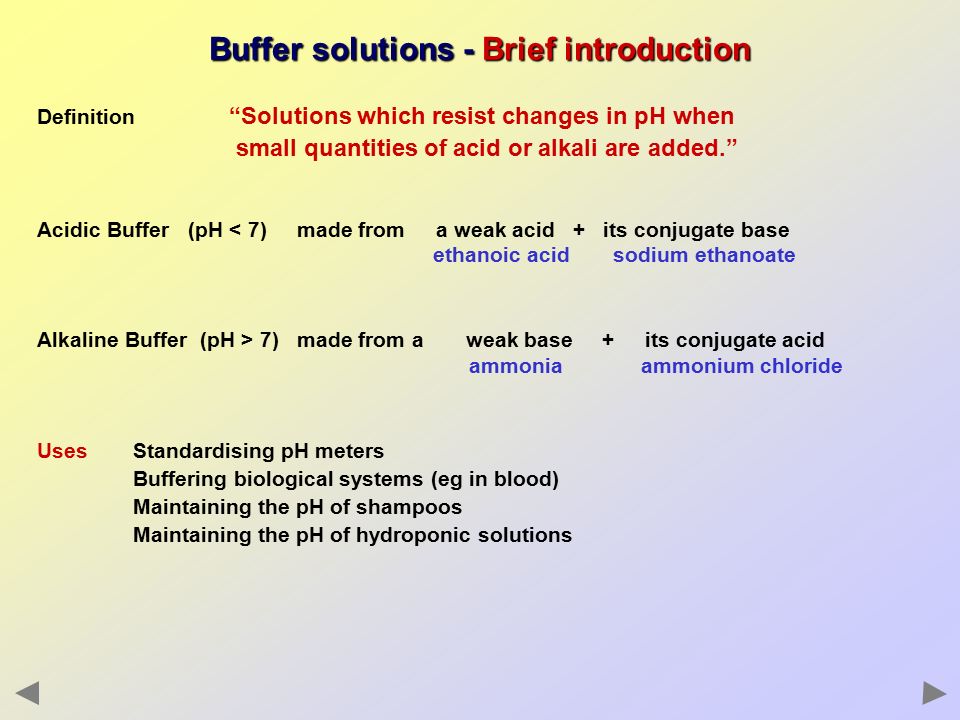
Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt. Buffer solutions are resistant to pH change because of the presence of an equilibrium between the acid HA and its conjugate base A. For example the bicarbonate buffering system is used to regulate the pH of blood.
Many life forms have a relatively small pH range. Buffer Solution is a water solvent based solution which consists of a mixture containing a weak acid and the conjugate base of the weak acid or a weak base and the conjugate acid of the weak base. Buffer solution and examples in chemistry.
I Acetic acidsodium acetate. An acidic buffer solution is simply one which has a pH less than 7. These ions are capable of binding extra H ions floating around in the blood.
When a solution experiences acid or base addition the pH will change drastically but in the presence of this buffer solution adding acid or base to the solution will only slightly change the pH of the solution and the change is not. The normal pH of human blood is 74. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. For example a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 475. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa.
For example blood contains a carbonic acid H 2 CO 3-bicarbonate HCO 3- buffer system. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
For example blood in the human body is a buffer solution. Buffer in chemistry solution usually containing an acid and a base or a salt that tends to maintain a constant hydrogen ion concentration. Its pH changes very little when a small amount of strong acid or base is added to it and is thus used to prevent a solution s pH change.
Within cells protein buffer systems are present to maintain a neutral pH. Buffer solutions are used in a wide variety of chemical applications. In this system the weak acid dissociates to a small extent giving bicarbonate ions.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature there are many systems that use buffering for pH regulation. Assuming the change in volume when the sodium acetate is not significant estimate the pH of the acetic acidsodium acetate buffer solution.
Buffers are broadly divided into two types acidic and alkaline buffer solutions. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. Mixture of acetic acid CH 3 COOH and Sodium acetate CH 3 COONa in water.
Iii Bicarbonatecarbon dioxide carbonic acid iv dihydrogen phosphatebiphosphate. In other words a buffer is an aqueous solution of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is composed of a weak acid and its conjugate base in appreciable concentrationsand so five examples are.
In this section we will examine some of the specific buffer systems throughout the body. Example Buffer Systems in the Body. Ions are atoms or molecules that have lost or gained one or more electrons.
Buffer solutions may be of two types. Its pH changes very little when a small amount of strong acid or base is added to it. An example of a buffer solution is blood.
An example of such a buffer is a mixture of ammonium hydroxide and ammonium chloride in water. Some people suffer from alkalosis when experiencing severe anxiety. Alkalosis is a condition in which the pH of the blood is too high.
A buffer solution is a fluid that opposes changes in pH when a little measure of corrosive or soluble base is included. Acidic buffers are solutions that have a pH below 7 and contain a weak acid and one of its salts. A buffer solution is a solution containing weak acids and their conjugate bases or weak bases and conjugated acids that are resistant to pH changes.
Buffers Indicators Acids And Bases 101 The Basics Of Chemistry
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 Buffer Solutions Biochemistry The Biology Notes
Buffer Solutions Biochemistry The Biology Notes
 Buffer And Isotonic Solution Ppt Video Online Download
Buffer And Isotonic Solution Ppt Video Online Download
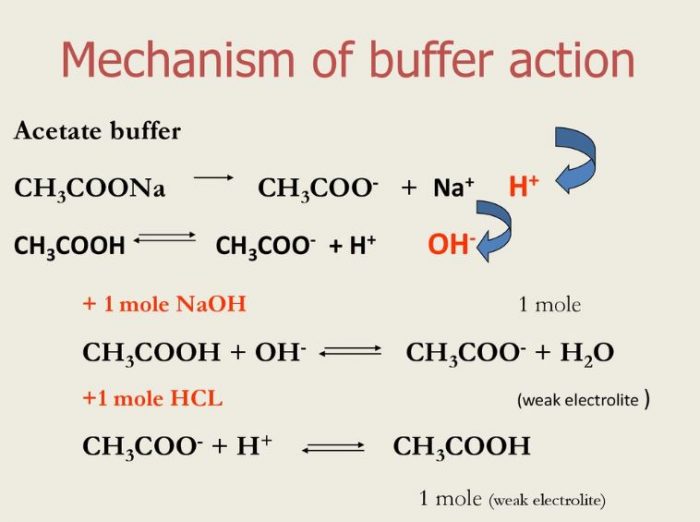 Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium
Buffer Solution Solution Of Reserve Acidity Alkalinity
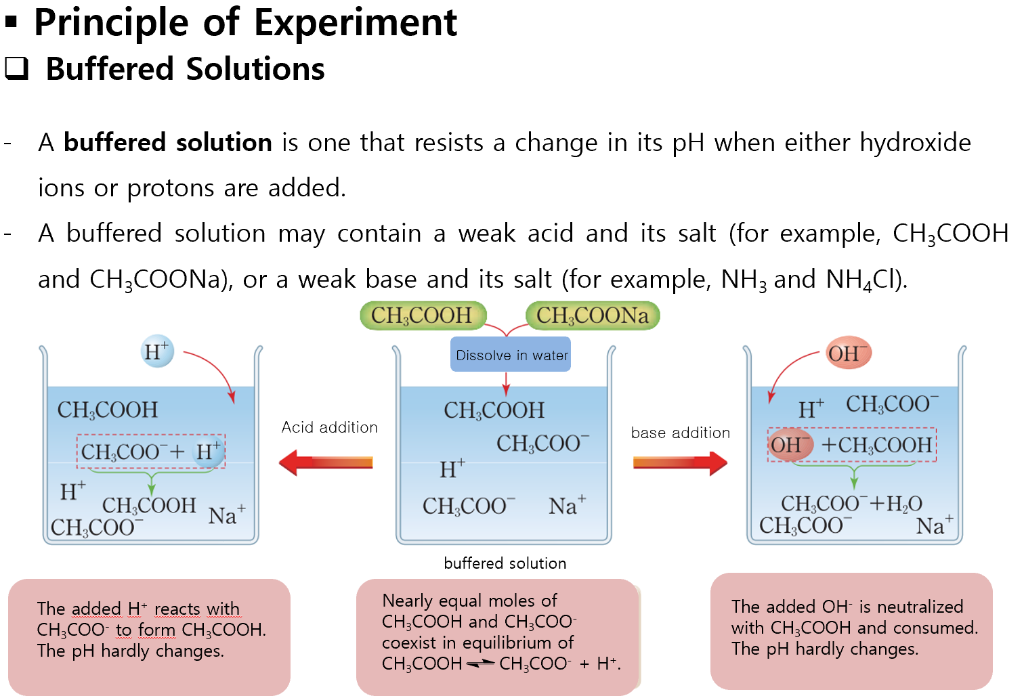 Solved Preparation Of Buffer Solution Experiment I Uploa Chegg Com
Solved Preparation Of Buffer Solution Experiment I Uploa Chegg Com
 Examples Of Buffer Solution In Everyday Life Their Applications And Uses
Examples Of Buffer Solution In Everyday Life Their Applications And Uses
 A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo
A Buffered Vs A Non Buffered Solution Chem13 News Magazine University Of Waterloo
What Is The Role Of A Buffer In A Pharmacy Quora
 Buffer Solution Types Of Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Youtube
Buffer Solution Types Of Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Youtube
 Buffers Definition Overview Expii
Buffers Definition Overview Expii
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
 18 2 1 Describe The Composition Of A Buffer Solution And Explain Its Action Youtube
18 2 1 Describe The Composition Of A Buffer Solution And Explain Its Action Youtube
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations